|
Ant surveys are extensively used to guide conservation decisions and form part of a ‘shopping basket’ of invertebrate taxa proposed for the use in monitoring programmes in South Africa’s national parks. However, very few ant inventories exist for these conservation areas. We report on the first quantitative survey of ants in the Marakele National Park (67 000 ha). Ants were sampled in four habitats, covering both the altitudinal range (1000 m a.s.l. – 2000 m a.s.l.) and three vegetation types in the park. A total of 4847 specimens, representing 29 genera and 104 species, were recorded from pitfall traps over a five-day period. Myrmicinae was the most abundant and diverse subfamily, representing 82% of all ants sampled, followed by the Formicinae subfamily, which represented 18% of the total abundance. The most abundant species were members of the Pheidole megacephala group, Pheidole sculpturata Mayr and members of the Monomorium salomonis group. In general, we found that the less complex habitats supported higher ant diversity. The Marakele National Park contains a quarter of the ant species recorded in South Africa and is a potential hotspot for invertebrate conservation.Conservation implications: The Marakele National Park represents an area of high ant – and therefore invertebrate – diversity. Ant conservation would require attention to each of the vegetation types to maintain complementarity (beta diversity) of the assemblages as well as consideration to the impact of large herbivores, whose presence positively influence ant richness at a site (alpha diversity).
The lack of taxonomic invertebrate expertise poses a significant challenge to conservation-related decision making. This statement is particularly relevant to ants (Hymenoptera: Formicidae): although traditionally considered a ’difficult‘ group (Bolton 1984), they are increasingly being used for environmental impact assessments (Andersen 1997), monitoring environmental change (Kaspari & Majer 2000; McGeoch & Sithole 2009) and even as surrogates for insect species richness (Uys, Hamer & Slotow 2010).Ant surveys are generally considered to be a very rich source of data for conservation planning and management (Kremen et al. 1993; Yek et al. 2009). They can also be used to delineate biogeographic zones, areas of high general biodiversity and centres of evolutionary radiation (Kremen et al. 1993). Ant monitoring systems have been developed in Australia to assess restoration success after mining and have since been applied successfully in assessing grazing impacts in rangelands (Andersen et al. 2004) and in the Succulent Karoo (Seymour & Dean 1999). Ant community data have been used predominantly to understand structural diversity in ecosystems, yet may prove to be seminal in studies attempting to answer important questions concerning functional biodiversity (Folgarait 1998). Ants are used as surrogates of ecosystems per se, because they form a staggeringly diverse and abundant group and constitute a substantial part of animal biomass (Hölldobler & Wilson 1994). Ants have invaded almost all ecosystems across the world; they are important ecosystem engineers (Samways 2005) and are significant predators of other terrestrial invertebrates (Hölldobler & Wilson 1994). There are also different functional groups amongst ants, displaying a wide range of life histories and food preferences (Andersen 2000). They perform important ecosystem functions, for example myrmecochory (Giliomee 2003; Lingyel et al. 2009) and the maintenance of rare lycaenid butterfly populations (Pierce et al. 2002). Above all, they are important ecosystem engineers. A considerable body of work exists on the ants of Africa (Arnold 1924; Fisher 2004; Garcia et al. 2009; Koch & Vohland 2004; Lévieux 1972; Marsh 1986; Taylor 2005; Willis, Skinner & Robertson 1992). African ant community richness clearly rivals that of other tropical ecosystems worldwide. Fisher (2004) collected a total of 310 species across 56 genera on Mont Doubou in Gabon. In recent years, surveys of epigaeic ants in South Africa have yielded interesting baseline data that indicate effects of agricultural activity (Addison & Samways 2000; Gaigher 2008), invasions by alien plants and the Argentine ant (Schoeman & Samways 2011), fire regimes (Parr, Bond & Robertson 2002; Parr & Chown 2003), vegetation types (Boonzaaier, McGeoch & Parr 2007) and predicted future climate change (Botes et al. 2006; Koch & Vohland 2004) on ant community assemblages. With such superdiverse assemblages, there are still gaps in our knowledge on the biology, ecology, taxonomy and distribution of ants in Africa. McGeoch et al. (2011) have recently identified five major groups that can serve as surrogates in conservation planning and monitoring, namely Araneae, Scarabaeidae (Coleoptera), Lepidoptera, Odonata and Formicidae (Hymenoptera). We report on an invertebrate study in the Marakele National Park, on the south-western border of the Limpopo province. The study aimed to determine ant diversity and generate baseline data that can serve as a reference for future studies in the Marakele National Park. Although there are several type specimens from the Waterberg Plateau (Gardiner & Terblanche 2010; Szüts & Jocqué 2001), this study probably represents only the second over-arching invertebrate survey for this protected area, the first being a study on termites (Isoptera) by Sileshi et al. (2010). The information from this study will contribute to the knowledge on invertebrates occurring across the network of South African protected areas.
The Marakele National Park (67 000 ha) is a sanctuary situated in the Waterberg region near the town of Thabazimbi in the Limpopo province, South Africa (Figure 1). It is a core conservation area in the Waterberg Biosphere Reserve. The park includes the highest point in the Limpopo province (2110 m a.s.l.). It receives 500 mm – 750 mm rainfall per year and includes three vegetation types (Mucina & Rutherford 2006), namely Western Sandy Bushveld, Waterberg Mountain Bushveld and Waterberg/Magaliesberg Summit Sourveld (Figure 1). The first two vegetation types mentioned are poorly protected in South Africa (Mucina & Rutherford 2006).
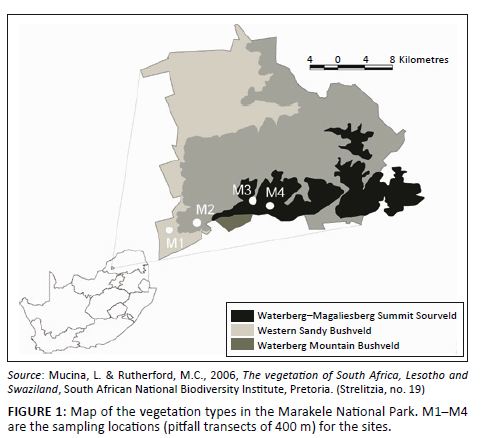 |
FIGURE 1: Map of the vegetation types in the Marakele National Park. M1–M4
are the sampling locations (pitfall transects of 400 m) for the sites.
|
|
Ants were sampled as part of the South African National Survey of Arachnida (SANSA). The SANSA protocol (Dippenaar-Schoeman & Craemer 2000) includes pitfall trapping as one of its sampling methods and requires that four sites be selected to include vegetation types representative of a survey area as a whole. Sites at the Marakele National Park were located at various altitudes along the western aspect of the mountain, namely at 1074 m (M1), 1182 m (M2), 1587 m (M3) and on the summit at 2044 m (M4), and in each of the vegetation types in the park (Figure 2a–d). One site, M3, was ecotonal (Figure 1 and Figure 2c). A total of 24 pitfall traps (diameter = 6 cm; depth = 10 cm) were placed in different patches at each site in February 2010. The traps were placed 20 m apart, flush with the soil surface, and care was taken not to disturb any existing leaf litter layers or nearby organic material in order to minimise digging-in effects (Greenslade 1973). The traps contained 50 mL propylene glycol, which is non-lethal and does not have a scent that can attract or repel ants. The pitfalls were left out for a period of 5 days. Pitfall samples from each site were pooled.
 |
FIGURE 2: Vegetation type at the four sampling sites. (a) M1, Western Sandy Bushveld; (b) M2, Waterberg Mountain Bushveld; (c) M3, ecotone on Waterberg–Magalies
Summit Sourveld and Waterberg Mountain Bushveld; (d) M4, Waterberg–Magalies Summit Sourveld.
|
|
Methods to process ants are described in Lattke (2000). Sampled ants were washed to remove any rubble, leaves and soil. Ants were then placed in 70% alcohol (96% alcohol diluted with glycol) and then sorted according to morphospecies. These were further separated and identified to genus, subgenus, species group or species level according to the classifications by Agosti and Johnson (2005), Bolton (1984), Hölldobler and Wilson (1994) and Taylor (2005). Agosti and Johnson (2005) and Taylor (2005) have a complete list of taxonomic references. A reference collection is housed in the Arthropod Collection of the Department of Zoology at the University of Venda.Species richness estimates and inventory completeness were calculated using Chao 1, a non-parametric technique based on the distribution of individuals amongst species (Colwell & Coddington 1994): 
where Sobs is the observed species, F1 the number of observed species represented by a single individual and F2 the number of observed species represented by more than one individual. With increasing pitfalls added to a sample, the observed species richness usually approaches the estimated species richness, which is depicted graphically. As we did not have replicates for a site, we used the Chao1 estimator and calculated inventory completion by comparing the estimated species richness with the observed richness per site. Simpson’s diversity index is one of many diversity indices that calculate a measure of diversity for communities based on the number of species represented and also their relative abundances. A diversity index was calculated for each site according to 
where N is the community size (i.e. Σn) and n is each population size. Species turnover between sites was calculated from square-root transformed abundance data using the Bray–Curtis similarity and hierarchically clustered using Primer 6 (Primer-E, Plymouth) (Figure 3).
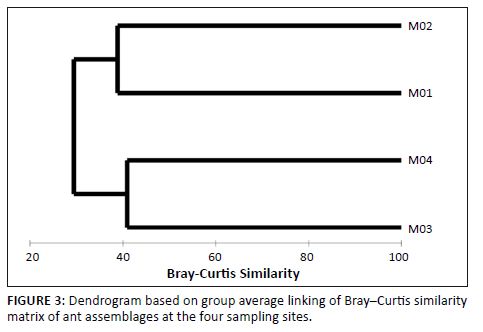 |
FIGURE 3: Dendrogram based on group average linking of Bray–Curtis similarity
matrix of ant assemblages at the four sampling sites.
|
|
A total of 4847 ants were collected. The ants were most abundant at the site of lowest altitude (M1), with 1737 individuals, followed by 1512 individuals collected at M3, 823 individuals at M2, and 775 individuals at M4. A total of 104 species across 29 genera were sampled (Tables 1 and 2), of which 18 were singletons that likely represent rare species and are not merely a result of undersampling. Observed species richness for all samples approached 98% of the Chao 1 species richness estimates. Results for separate sites also show that sampling was relatively complete, with observed species richness approaching 89%, 98%, 96% and 97% of the estimated species richness for M1, M2, M3 and M4, respectively. The use of pitfall sampling allowed us to collect strictly epigaeic groups. The ant fauna profile could be completely different with another sampling method. Winkler traps may, for example, give different results with a very distinct fauna.Diversity differed between sites, as shown by Simpson’s diversity index: M3, the ecotonal site, was the most diverse (54 species, n = 1512, D = 3.48) followed by M1 (42 species, n = 1737, D = 2.33), whilst M2 (40 species, n = 823, D = 1.48) and M4 (35 species, n = 775, D = 177) had lower diversity than the other sites. Five subfamilies were collected: Ponerinae, Dolichoderinae, Dorylinae, Formicinae and Myrmicinae. Myrmicinae was the most abundant and most diverse subfamily sampled (63 species, n = 4214), comprising 87% of ant abundance and 61% of all species. Formicinae was the next most diverse and abundant subfamily (19 species, n = 364), comprising 18% of the total number of ant species sampled (Table 1). The least represented subfamilies were Dolichoderinae, with six species of Tapinoma, and Dorylinae, with three species of Dorylus.
|
TABLE 1: Species richness and abundance of ant subfamilies collected at the Marakele National Park, Limpopo province, South Africa.
|
As shown in Table 2, the most abundant Myrmicinae species were Pheidole sp. 1 megacephala group (n = 1137), Pheidole sp. 2 capensis group (n = 147), Pheidole sculpturata (n = 486), Tetramorium quadrispinosum Emery (n = 208) and Monomorium sp. 4 salomonis group (n = 7780). All these species were common to all the sites. Other species that were not so abundant but still occurred across all or most sites were Monomorium sp. 3 destructor group, Pheidole sp. 2 rugaticeps group, Solenopsis sp. 4 and Tetramorium sp. 7 similimum group, which is a tramp species that occurred in many different habitats. Similarly, T. quadrispinosum co-occurred with the invasive Argentine ant (Linepithema humile) in studies of plant invasions in the Western Cape (Schoeman & Samways 2011).
|
TABLE 2: Checklist of ants of the Marakele National Park, Limpopo province,
South Africa.
|
Many species seemed to be localised or restricted to one habitat or altitude, for example Calyptomyrmex clavatus, Cardiocondyla emeryi, Messor denticornis and Myrmicaria fusca, as well as some highly localised Monomorium, Pheidole and Tetramorium species. The most abundant Formicinae ants were Anoplolepis custodiens (n = 35), Camponotus sp. 8 rufoglaucus group (n = 125), Lepisiota capensis (n = 38) and Paratrechina sp. 1 (n = 49). Of these, Paratrechina sp. 1 was the only species restricted to M3. Localised species were less abundant and included Camponotus (Myrmacrhaphe) sp. 1, Camponotus mayri, Lepisiota rubrovaria group (both species sampled) and Plagiolepis puncta (Table 2). All species of the Dorylinae and Dolichoderinae subfamilies were sampled only in M3, with the exception of Tapinoma voeltzkowi Forel (found at all sites), Tapinoma sp. 2 and Dorylus (Dorylus) sp. 1. The most abundant ponerine ant was Hypoponera similoponera group (n = 102). The remaining ponerine ants were restricted to single sites, with the exception of Pachycondyla granosa, which was found in two sites. Ponerinae species are generally rarer than other ants, but in the Marakele National Park the assemblage seems to combine rarity with diversity (12 species, n = 173). It is also worth noting that both the known species of Odontomachus occur in the park. Some ant species were sampled only in specific vegetation types, indicating that the ant assemblages here differ between the different habitats. Nearly half of all sampled species (n = 56; 53%) were restricted to a single site. The percentages of species sampled only in M1, M2, M3, and M4 were 38%, 38%, 33% and 20%, respectively. Considerable variation in species assemblage composition was found between sites, with no one site sharing more than 39% of the species. M1 and M2 clustered together with a Bray–Curtis similarity of 39, whilst M3 and M4 formed a group based on a similarity of 41 (Figure 3). M2 and M4 were the least similar (similarity = 25). There was a clear distinction between high (M3 and M4) and low (M2 and M1) elevational ant assemblages according to latitude.
Although sampling lasted only 5 days, it was across a representative altitudinal range and vegetation types occurring in the park. The survey therefore approximates most of the ant genera in the park. Inventory completion was satisfactory and suggests that sampling effort was sufficient for the habitats surveyed. Approximately 415 ant species are known in South Africa (Taylor 2005), although there are certainly more. The Marakele National Park, which covers a land surface of 67 000 ha, hosts approximately a quarter of these species. It is probable that some species found in this study have not previously been recorded in South Africa. This observation compares particularly well with the situation in the Kruger National Park, where 162 species have been recorded over an extended period (Parr et al. 2004). The species richness also compares well with that of an altitudinal transect (160 km) in the Cederberg mountains in the Western Cape (Botes et al. 2006), in which 86 species were recorded using substantially more intensive sampling (> 50 000 specimens).The survey reported here provides comprehensive baseline data across the altitudinal sweep and vegetation types at the Marakele National Park. Based on the low Bray–Curtis similarities (i.e. < 40), there is considerable turnover between sites. This highlights the importance of conserving different vegetation types for the maintenance of ant diversity in Marakele. The two most diverse sites were M1 and M3. M1 was vegetationally complex, with more trees and more canopy cover than the other (Figure 2). M3 was situated on an ecotone between Waterberg Mountain Bushveld and Waterberg–Magalies Summit Sourveld (Figure 2), which may account for the high diversity at this particular site. This suggests that the physical structure of the vegetation (and hence habitat) may play a role in promoting greater ant species richness (Lubertazzi & Tschinkel 2003). However, results obtained by Lassau and Hochuli (2004) showed that ant species richness was negatively associated with ground
herb cover, tree canopy cover, soil moisture and leaf litter. During the course of sampling, we also noticed that there was a greater degree of large herbivore trampling in these sites (M1 for instance was close to a rhino latrine). Other studies have shown that large herbivore trampling stimulates invertebrate diversity by creating a more heterogeneous habitat (Tscharntke & Greiler 1995). M2 and M4 yielded a lower species richness than the other sites. The low diversity at M4 may be accounted for by the fact that it is situated at the highest peak in the Waterberg. Studies have shown that diversity decreases with increasing elevation (McCoy 1990) owing to a decrease in net primary productivity. For the current study, however, there seems to be no clear relationship between elevation and diversity, as our most diverse site was located at 1587 m a.s.l., being the third highest site in the study. With regard to community composition and similarity, M1 and M2, and M3 and M4 clustered together, respectively (Figure 3). However, as stated before, the turnover between these sites is considerable. We could not deduce any discernable patterns of higher taxonomic level dissimilarity between the sites, except for M4, which displayed reduced Myrmicinae ant species richness, specifically with reference to Monomorium species. M3 displayed a greater diversity and abundance of Dolichoderinae and Dorylinae ants than the other sites, where these subfamilies were poorly represented. So far, though, studies indicate that the physical structure of the habitat can have a distinct effect on composition of the ant communities, affecting niche differentiation and distribution within these habitats (Legendre, Borcard & Peres-Neto 2005). In Marakele, the presence of large herbivores, combined with vegetational complexity, plays a role in the actual generation of ant species richness within sites, whilst community composition here is largely determined by vegetation type. More realistically, ant community assemblages function as important ecosystem engineers that change a habitat whilst being changed by the habitat in a functionally changing ecosystem. The survey was conducted over a relatively short period using only one sampling technique and therefore our data may not be a true reflection of the total ant species diversity in the park. Further studies should include long-term monitoring at a site and management objectives in design of the sampling protocol. Surveys of epigaeic ants suggest that abundances and assemblage composition differ between biomes not only as a result of change in vegetation (Botes et al. 2006) but also in response to net primary productivity (Kaspari, O’Donnell & Kercher 2000). Whether ant community composition differs between vegetation types or units within a biome still needs to be determined; thus far our results suggest that this may be the case. However, we caution that previous studies have shown that differences in invertebrate community assemblage of replicates in the same vegetation type might differ more from each other than assemblages in other vegetation types in the same biome (Muelelwa et al. 2010). Our assumptions of what exactly constitutes a habitat would therefore need reassessment.
Our results suggest that the Marakele National Park, and by extension the Waterberg region, is a hotspot for ant diversity in South Africa. It may be worth testing whether the groups identified by McGeoch and Sithole (2009) can act as surrogates for one another in any projected future monitoring programmes. To this end, detailed and freely available inventories of ants, butterflies, dragonflies and scarab beetles need to be compiled for all protected areas in South Africa. So far, an inventory of spiders has been compiled as part of SANSA. Such inventories will not only make a significant contribution to existing knowledge of insects in South Africa’s protected areas, but will also facilitate movement towards full-scale use of invertebrates in monitoring and other conservation activities.
We would like to thank the management of the Marakele National Park for allowing us to conduct this survey, Norbert Hahn and Ovavhu Gelebe for help with field work, and SANSA and the Research and Publications Committee (University of Venda) for financial support. S.H.F. acknowledges support from the DST-NRF Centre of Excellence for Invasion Biology.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this paper.
Authors’ contributions
C.S.S. (University of Venda) was responsible for preparation and identification of specimens and all calculations. S.H.F. (University of Venda) was the project leader and made conceptual contributions to the manuscript.
Addison, P. & Samways, M.J., 2000, ‘A survey of ants (Hymenoptera: Formicidae) that forage in vineyards in the Western Cape Province, South
Africa’, African Entomology 8(2), 251−260.Agosti, D. & Johnson, N.F. (eds.), 2005, Antbase, viewed 13 March 2010, from antbase.org (version 05/2005). Andersen, A.N., 1997, ‘Using ants as bioindicators: Multiscale issues in ant community ecology’, Conservation Ecology 1(1), 8, viewed
24 November 2011, from
http://www.consecol.org/vol1/iss1/art8
Andersen, A.N., 2000, ‘A global ecology of rainforest ants: functional groups in relation to environmental stress and disturbance’, in D. Agosti,
J.D. Majer, L.E. Alonso & T.R. Schultz (eds.), Ants: Standard methods for measuring and monitoring biodiversity, pp. 25–34, Smithsonian Institution
Press, Washington DC. Andersen, A.N., Fisher, A., Hoffman, B.D., Read, J.L. & Richards, R., 2004, ‘Use of terrestrial invertebrates for biodiversity monitoring in
Australian rangelands, with particular reference to ants’, Austral Ecology 29, 87−92.
http://dx.doi.org/10.1111/j.1442-9993.2004.01362.x Arnold, G., 1924, ‘A monograph of the Formicidae of South Africa. Part 6. (Camponotinae)’, Annals of the South African Museum 14, 675–766. Bolton, B., 1984, Identification guide to ant genera of the world, Harvard University Press, Cambridge, MA. Boonzaaier, C., McGeoch, M.A. & Parr, C.L., 2007, ‘Fine-scale temporal and spatial dynamics of epigaeic ants in Fynbos: sampling implications’,
African Entomology 15(1), 1−11. http://dx.doi.org/10.4001/1021-3589-15.1.1 Botes, A., McGeoch, M.A., Robertson, H.G., Van Niekerk, A., Davids, H.P. & Chown, S.L., 2006, ‘Ants, altitude and change in the northern Cape
Floristic Region’, Journal of Biogeography 33, 71−90.
http://dx.doi.org/10.1111/j.1365-2699.2005.01336.x Colwell, R.K. & Coddington, J.A., 1994, ‘Estimating terrestrial biodiversity through extrapolation’, Philosophical Transactions of the
Royal Society of London B 345, 101−108. http://dx.doi.org/10.1098/rstb.1994.0091,
PMid:7972351
Dippenaar-Schoeman, A.S. & Craemer, C., 2000, ‘The South African National Survey of Arachnida’, Plant Protection News 56, 11−12. Fisher, B.L., 2004, ‘Diversity patterns of ants (hymenoptera: formicidae) along an elevational gradient on Monts Doudou in southwestern Gabon’,
California Academy of Sciences Memoir 28, 269–286. Folgarait, P.J., 1998, ‘Ant biodiversity and its relationship to ecosystem functioning: a review’, Biodiversity and Conservation 7(9),
1221−1244. http://dx.doi.org/10.1023/A:1008891901953 Gaigher, R., 2008, ‘The effect of different vineyard management systems on the epigaiec arthropod assemblages in the Cape Floristic Region, South
Africa’, MSc thesis, Dept. of Conservation Ecology and Entomology, University of Stellenbosch. Garcia, F.H., Fischer, G., Peters, M.K., Snelling, R.R. & Wägele, J.W., 2009, ‘A preliminary checklist of the ants (Hymenoptera: Formicidae) of
Kakamega Forest (Kenya)’, Journal of East African Natural History 98(2), 147–165. Gardiner, A.J. & Terblanche, R.F., 2010, ‘Taxonomy, biology, biogeography, evolution and conservation of the genus Eriksonia Trimen
(Lepidoptera: Lycaenidae)’, African Entomology 18(1), 171−191. http://dx.doi.org/10.4001/003.018.0114 Giliomee, J.H., 2003, ‘Insect diversity in the Cape Floristic Region’, African Journal of Ecology 41(3), 237−244.
http://dx.doi.org/10.1046/j.1365-2028.2003.00442.x Greenslade, P.J.M., 1973, ‘Sampling ants with pitfall traps: digging-in effects’, Insectes Sociaux 20(4), 343–353.
http://dx.doi.org/10.1007/BF02226087 Hölldobler, B. & Wilson, E.O., 1994, The ants, Springer-Verlag, Berlin. Kaspari, M. & Majer, J.D., 2000, ‘Using ants to monitor environmental change’, in D. Agosti, J.D. Majer, L.E. Alonso & T.R. Schultz
(eds.), Ants: Standard methods for measuring and monitoring biodiversity, pp. 89–98, Smithsonian Institution Press, Washington DC. Kaspari, M., O’Donnell, S. & Kercher, J.R., 2000, ‘Energy, density, and constraints to species richness: ant assemblages along a productivity
gradient’, The American Naturalist 155, 280−293. http://dx.doi.org/10.1086/303313,
PMid:10686166
Koch, F. & Vohland, K., 2004, ‘Ants along a southern African transect - a basis for biodiversity change monitoring (Insecta, Hymenoptera, Formicidae)’, Zoosystematics and Evolution 80(2), 261–273. Kremen, C., Colwell, R.K., Erwin, T.L., Murphy, D.D., Noss, R.F. & Sanjayan, M.A., 1993, ‘Terrestrial arthropod assemblages: Their use in conservation
planning’, Conservation Biology 7(4), 796−808.
http://dx.doi.org/10.1046/j.1523-1739.1993.740796.x Lassau, S.A. & Hochuli, D.F, 2004, ‘Effects of habitat complexity on ant assemblages: Can we generalise across scale?’, Ecography 27(2),
157−164. http://dx.doi.org/10.1111/j.0906-7590.2004.03675.x Lattke, J.E., 2000, ‘Specimen processing: building and curating an ant collection’, in D. Agosti, J.D. Majer, L.E. Alonso & T.R. Schultz (eds.),
Ants: Standard methods for measuring and monitoring biodiversity, pp. 155–171, Smithsonian Institution Press, Washington DC. Legendre, P., Borcard, D. & Peres-Neto, P.R., 2005, ‘Analyzing beta diversity: partitioning the spatial variation in community composition data’,
Ecological Monographs 75(4), 435–450. http://dx.doi.org/10.1890/05-0549 Lévieux, J., 1972, ‘Le rôle des fourmis dans les réseaux trophiques d’une savane préforestière de Côte
d’Ivoire [The role of ants in a trophic network in a preforested savanna in the Ivory Coast]’, Annales de l’Université d’Abidjan
ser. E, 5, 143–240. Lingyel, S., Gove, A.D., Latimer, A.M., Majer, J.D. & Dunn, R.R., 2009, ‘Convergent evolution of seed dispersal by ants, and phylogeny and biogeography
in flowering plants: a global review’, Perspectives in Plant Ecology, Evolution and Systematics 12(1), 43−55.
http://dx.doi.org/10.1016/j.ppees.2009.08.001 Lubertazzi, D. & Tschinkel, D.R., 2003, ‘Ant community change across a ground vegetational gradient in north Florida’s longleaf pine
flatwoods’, Journal of Insect Science 3, 21, viewed 24 November 2011, from
http://www.insectscience.org/3.21,
PMid:15841237,
PMCid:524660
Marsh, A.C., 1986, ‘Checklist, biological notes and distribution of ants in the central Namib Desert’, Madoqua 14(4), 333–344. McCoy, E.D., 1990, ‘The distribution of insects along elevational gradients’, Oikos 58(3), 313–322, viewed 04 May 2011, from
http://www.jstor.org/pss/3545222 McGeoch, M.A., Sithole, H., Samways, M.J., Simaika, J.P., Pryke, J.S., Picker, M. et al., 2011, ‘Conservation and monitoring of invertebrates in
terrestrial protected areas’, Koedoe 53, Art. #1000.
http://dx.doi.org/10.4102/koedoe.v53i2.1000
Mucina, L. & Rutherford, M.C., 2006, The vegetation of South Africa, Lesotho and Swaziland, South African National Biodiversity Institute, Pretoria.
(Strelitzia, no. 19) Muelelwa, M.I., Foord, S.H., Dippenaar-Schoeman, A.S. & Stam, E.M., 2010, ‘Towards a standardized and optimized protocol for rapid biodiversity
assessments: Spider species richness and assemblage composition in two savanna vegetation types’, African Zoology 45(2), 273–290.
http://dx.doi.org/10.3377/004.045.0206
Parr, C.L. & Chown, S.L., 2003, ‘Burning issues in conservation: a critique of faunal fire research in Southern Africa’, Austral Ecology
28, 384–395. http://dx.doi.org/10.1046/j.1442-9993.2003.01296.x Parr, C.L., 2003, ‘Ant assemblages in a Southern African savanna: Local processes and conservation implications’, PhD thesis, Dept. of Entomology,
University of Pretoria. Parr, C.L., Bond, W.J. & Robertson, H.G., 2002, ‘A preliminary study of the effect of fire on ants (Formicidae) in a South African savanna’,
African Entomology 10, 101–111. Parr, C.L., Robertson, H.G., Biggs, H.C. & Chown, S.L., 2004, ‘Response of African savanna ants to long-term fire regimes’, Journal of
Applied Ecology 41(4), 630–632. http://dx.doi.org/10.1111/j.0021-8901.2004.00920.x Pierce, N.E., Braby, M.F., Heath, A., Lohman, D.J., Mathew, J., Rand, D.B. et al., 2002, ‘The ecology and evolution of ant association in the
Lycaenidae (Lepidoptera)’, Annual Review of Entomology 47, 733−771.
http://dx.doi.org/10.1146/annurev.ento.47.091201.145257,
PMid:11729090
Samways, M.J., 2005, Insect diversity conservation, Cambridge University Press, Cambridge.
http://dx.doi.org/10.1017/CBO9780511614163 Schoeman, C.S. & Samways, M.J., 2011, ‘Synergisms between the invasive alien Argentine ant and alien plants on native ants of the Western Cape,
South Africa’, African Entomology 19(1), 96–105. Seymour, C.L. & Dean, W.R.J., 1999, ‘Effects of heavy grazing on invertebrate assemblages in the Succulent Karoo, South Africa’, Journal of
Arid Environments 43, 267−286. http://dx.doi.org/10.1006/jare.1999.0552 Sileshi, G.W., Arshad, M.A., Konate, S. & Nkunika, P.O.Y., 2010, ‘Termite-induced heterogeneity in African savanna vegetation: mechanisms and
patterns’, Journal of Vegetation Science 21(5), 923−937.
http://dx.doi.org/10.1111/j.1654-1103.2010.01197.x Szüts, T. & Jocqué, R., 2001, ‘A revision of the Afrotropical spider genus Palfuria (Araneae: Zodariidae)’, Journal
of Arachnology 29, 205−219.
http://dx.doi.org/10.1636/0161-8202(2001)029[0205:AROTAS]2.0.CO;2 Taylor, B., 2005, The ants of (sub-Saharan) Africa, viewed 29 June 2010, from
http://antbase.org/ants/africa
Tscharntke, T. & Greiler, H.J., 1995, ‘Insect communities, grasses and grasslands’, Annual Review of Entomology 40, 535–558.
http://dx.doi.org/10.1146/annurev.en.40.010195.002535 Uys, C., Hamer, M. & Slotow, R., 2010, ‘Step process for selecting and testing surrogates and indicators of Afromontane forest invertebrate
diversity’, PLoS ONE 5(2), e1900, viewed on 03 October 2010, from
http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0009100 Willis, C.K., Skinner, J.D. & Robertson, H.G., 1992, ‘Abundance of ants and termites in the False Karoo and their importance in the diet
of the aardvark Orycteropus afer’, African Journal of Ecology 30(4), 322–334.
http://dx.doi.org/10.1111/j.1365-2028.1992.tb00509.x Yek, S.H., Williams, S.E., Burwell, C.J., Robson, S.K.A. & Crozier, R.H., 2009, ‘Ground dwelling ants as surrogates for establishing conservation
priorities in the Australian wet tropics’, Journal of Insect Science 12, 1−12.
http://dx.doi.org/10.1673/031.009.1201,
PMid:19613441,
PMCid:3011884
|